WASHINGTON DC (CLARKSVILLENOW) – The Federal Drug Administration (FDA) announced this week that a new ‘at home’ test kit for COVID-19 may soon be headed to stores.
The kit, created by LabCorp, was approved through an Emergency Use Authorization through the FDA.
“Throughout this pandemic we have been facilitating test development to ensure patients access to accurate diagnostics, which includes supporting the development of reliable and accurate at-home sample collection options,” said FDA Commissioner Stephen M. Hahn, M.D. “The FDA’s around-the-clock work since this outbreak began has resulted in the authorization of more than 50 diagnostic tests and engagement with over 350 test developers. Specifically, for tests that include home sample collection, we worked with LabCorp to ensure the data demonstrated from at-home patient sample collection is as safe and accurate as sample collection at a doctor’s office, hospital or other testing site. With this action, there is now a convenient and reliable option for patient sample collection from the comfort and safety of their home.”
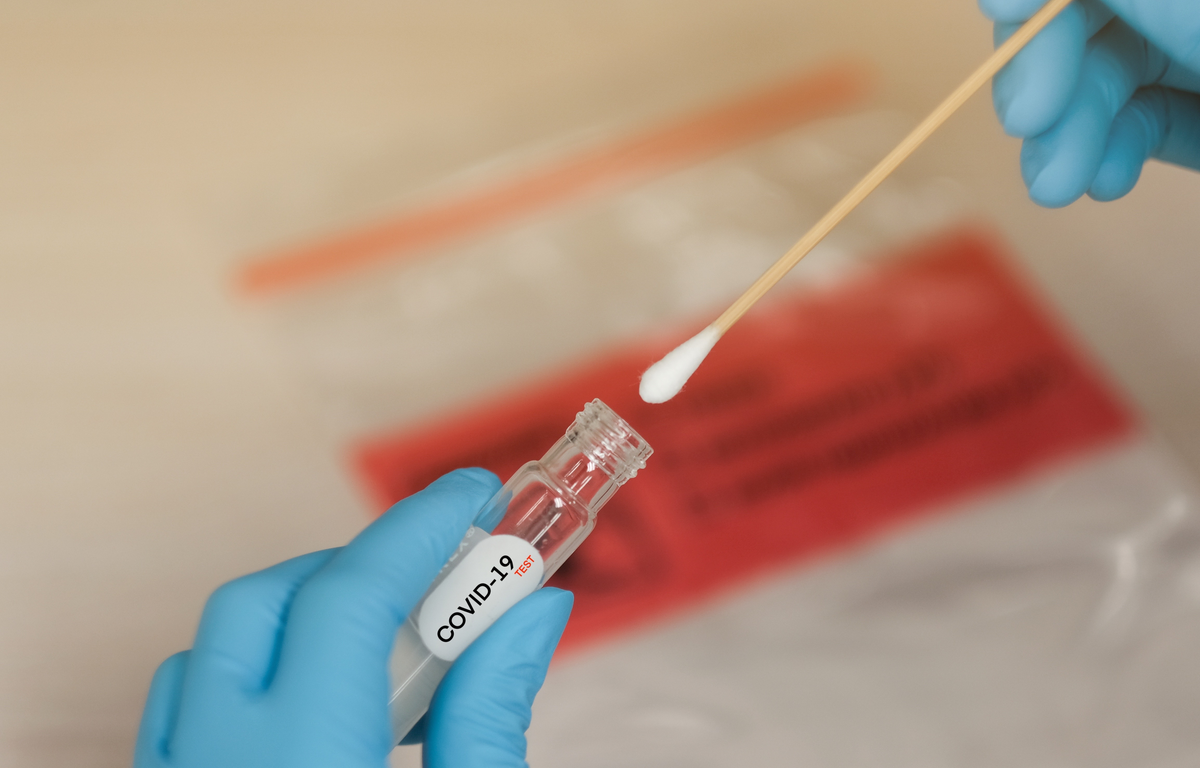
The patient administered test kit will provide designated nasal swabs and saline. Patients will self-swab to collect their nasal sample, then mail their sample, in an insulated package, to a LabCorp lab for testing. According to a statement from the company, “LabCorp intends to make the Pixel by LabCorp COVID-19 Test home collection kits available to consumers in most states, with a doctor’s order, in the coming weeks.”
The test is expected to cost around $119 and may be covered by most insurance providers.
The self-collection kit includes a specific Q-tip-style cotton swab for patients to use to collect their sample. “Due to concerns with sterility and cross-reactivity due to inherent genetic material in cotton swabs, other cotton swabs should not be used with this test at the present time,” cautions FDA officials.
Officials say the self-administered swabs will not be as ‘invasive’ as the clinician administered swabs. When administered by medical professionals, the swab is inserted deep into the nasal cavity, where the back of the nose meets the top of the throat. This self administered swab will not be required to adhere to the same process, instead being just a simple swab directly inside the nose.
The FDA will continue to work with test developers to determine whether or not Q-tip-style cotton swab can be used safely and effectively with other tests.