The Food and Drug Administration (FDA) announced a recall of a hypertension medication.
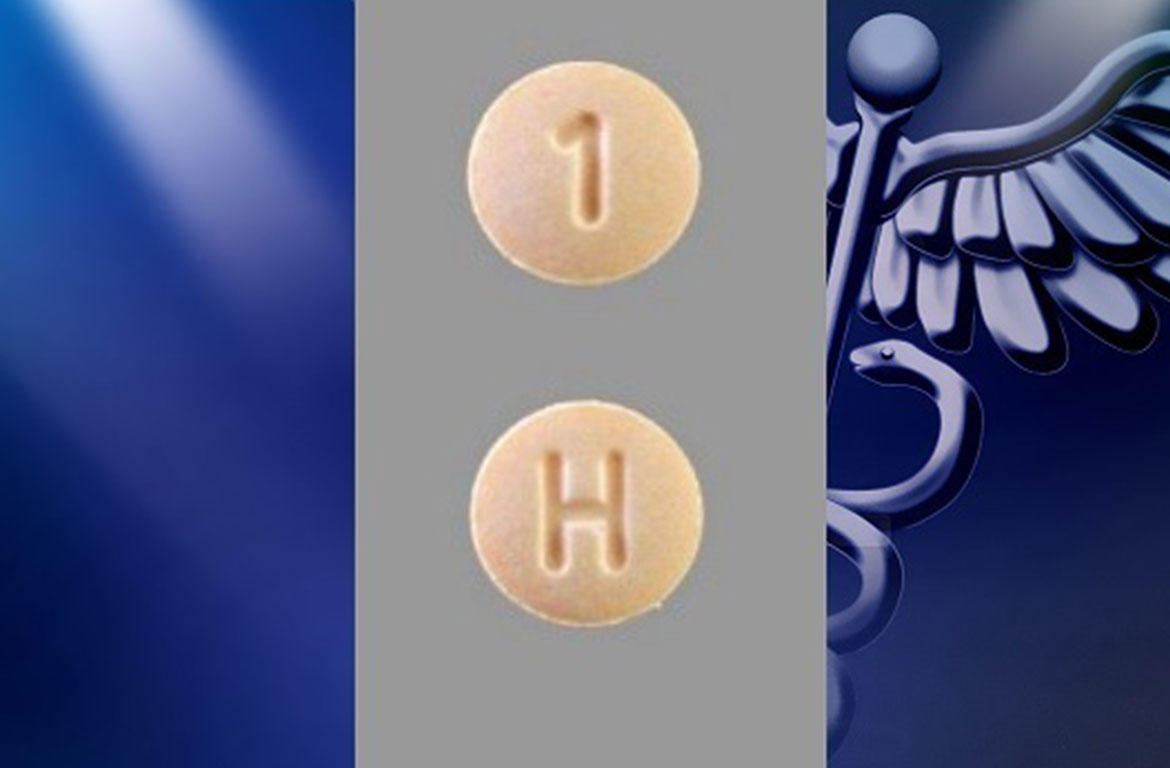
Accord Healthcare Inc. is voluntarily recalling One lot (Lot PW05264 – 46632 Bottles, NDC 16729-182-01) of Hydrochlorothiazide Tablets USP, 12.5 mg
A 100-count bottle has been found to contain 100 Spironolactone Tablets USP 25 mg as the result of a potential labeling error.
Accord believes that no other lots of Hydrochlorothiazide Tablets are involved in this mix-up. Accord became aware of this finding through a product complaint reported from a pharmacy.
Use of spironolactone tablets instead of hydrochlorothiazide tablets poses the risk of contracting hyperkalemia (increase potassium levels) in certain people.
To date, Accord has not received any reports of illness or adverse effects related to this recall.
Consumers/Pharmacies with questions regarding this recall can contact Accord Healthcare, Inc. by phone at 1-855-869-1081, fax: 1-817-868-5362 or e-mail at rxrecalls@inmar.com Monday to Friday during business hours 8 am to 5 pm EST. Consumers should contact their physician or healthcare provider if they have experienced any problems that may be related to taking or using this drug product.
You can find more info on the FDA’s website.