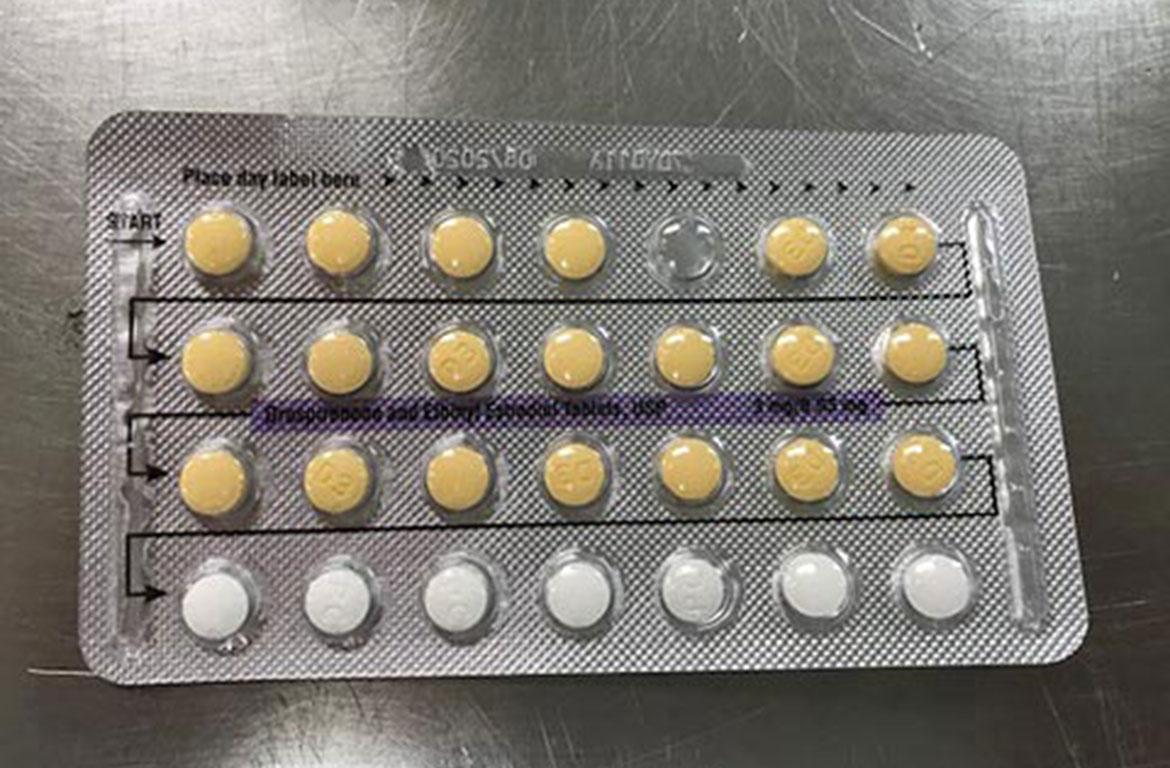
A Florida company is voluntarily recalling some birth control pills due to a packaging error, according to the FDA.
Weston, Florida, Apotex Corp. is voluntarily recalling four lots of Drospirenone and Ethinyl Estradiol Tablets, USP. These pills may have defective packaging with incorrect tablet arrangements and/or an empty blister pocket.
As a result of this packaging error, where a patient does not take a tablet due to a missing tablet or that a patient takes a placebo instead of an active tablet, the pills may not work to prevent pregnancy.
The company says that to date, no cases have been reported to Apotex.
Patients who have received impacted lots of Drospirenone and Ethinyl Estradiol Tablets, USP 3MG/0.03MG. or have questions regarding this recall are urged to contact their pharmacies and physicians.
For more information about the recall, visit the FDA’s website.